Peripartum Cardiomyopathy
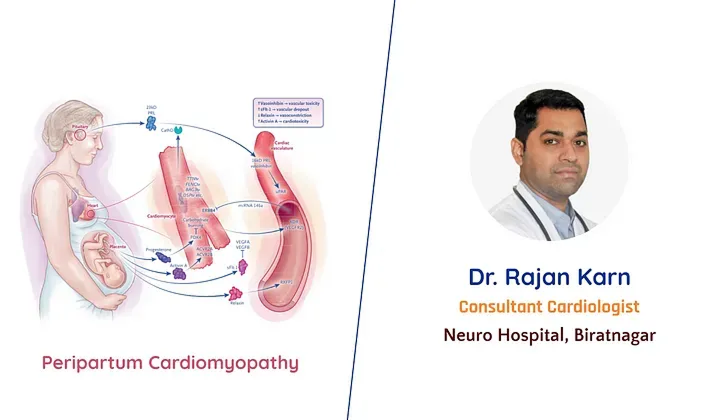
Peripartum cardiomyopathy (PPCM) is a form of acute and sometimes severe cardiac degeneration that leads to clinical heart failure during pregnancy or in the early postpartum period. The disorder is generally defined as maternal heart failure with systolic dysfunction (left ventricular ejection fraction, < 45%) that develops in the last month of pregnancy or in the first 5 months after delivery, in the absence of known pre-existing cardiac dysfunction.
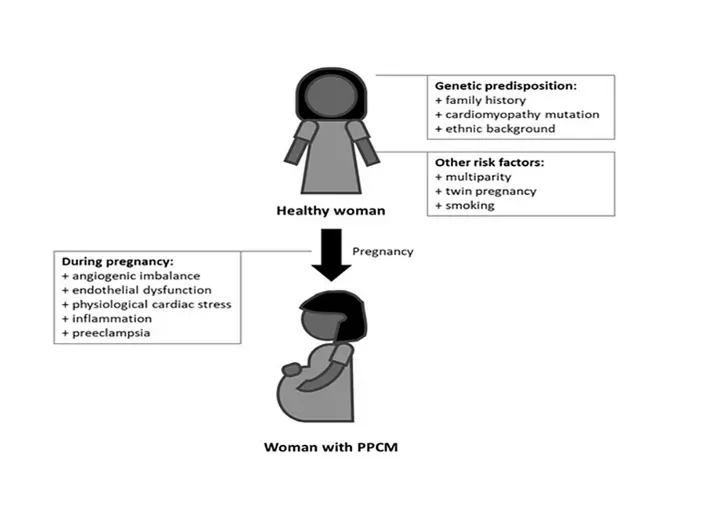
Peripartum cardiomyopathy complicates approximately 1 in 2000 births worldwide, with substantial variation among regions.PPCM is more common in women older than 30 years, black women, multiparous women, women with preeclampsia or hypertension, and those who smoke or are malnourished.
Peripartum cardiomyopathy is now a leading cause of maternal death around the world. Approximately 60% of cases of cardiogenic shock during pregnancy or in the early postpartum period are caused by peripartum cardiomyopathy. Although cardiac function typically recovers in more than 50% of affected patients, morbidity and mortality are nevertheless high, with some patients requiring a left ventricular assist device (LVAD) or cardiac transplantation.
Clinical Presentation
Women with PPCM most commonly present in the last month of pregnancy or in the first five months postpartum with symptoms of dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, edema of the lower extremities, fatigue, chest pain and cough. A few patients may also present in cardiogenic shock. A minority of patients present with severe arrhythmias. Physical examination may reveal pulmonary rales, raised jugular venous pressure, peripheral edema, tachypnea, and tachycardia with irregular peripheral pulses, displaced apical impulse, or murmurs of mitral and tricuspid regurgitation.
Diagnosis
- 12-lead electrocardiogram — Identifying associated arrhythmias
- Echocardiography- to confirm the diagnosis, to assess associated cardiac conditions and their severity
- Cardiac Biomarkers-for ruling out heart failure
- MRI-can accurately assess cardiac chamber volume and systolic function and is more sensitive in identifying intracardiac thrombus than echocardiography.
- Endomyocardial Biopsy- is rarely performed to exclude other causes of LV dysfunction, particularly when there is diagnostic uncertainty.
Etiology
There is evidence that various mechanisms may be responsible for PPCM, including hemodynamic stresses of pregnancy, vasculo-hormonal factors, inflammation, immunology and genetics. These remain plausible etiologies, but none of them is definitive since PPCM is primarily considered an idiopathic myocardial disease of pregnancy.
Management
Acute heart failure during pregnancy
Rapid diagnosis and decision-making are essential for all pregnant women with acute heart failure, as are a management algorithm and establishment of a multidisciplinary team that includes cardiologists, intensivists, obstetric physicians, neonatologists, anesthesiologists, and cardiac surgeons.
At the same time, the welfare of the fetus must be considered while implementing diagnostic tests and treatment during pregnancy. Therapeutic recommendations for mild, moderate, and severe forms of heart failure are listed under the BOARD therapeutic scheme (like Bromocriptine, Oral heart failure therapy, Anticoagulation, Vaso-relaxing agents, and Diuretics)
Women with severe forms of heart failure will require intensive care management, with invasive monitoring including intra-arterial blood pressure, central venous pressure, and a pulmonary artery catheter.
Women presenting with shock will require inotropic and vasopressor support. Patients refractory to medical management may require short-term assist devices in the form of extracorporeal membrane oxygenation or long-term devices such as a ventricular assist device (VAD).
Cardiac Transplantation
In the worst-case scenario, if all possible conservative therapeutic options fail, heart transplantation is the last treatment choice.
Delivery
Unless PPCM threatens the life of mother or fetus, there is no indication for early delivery, but onset of hemodynamic instability requires urgent delivery irrespective of gestation period.
A planned Cesarean section with appropriate inotropic/vasopressor and/or mechanical support is preferred in decompensated or very severe heart failure, while induction of labor may be possible in most asymptomatic women with less severe heart failure.
Epidural analgesia is recommended during labor as it will stabilize cardiac output, while combined spinal and epidural anesthesia is preferred for Cesarean sections.
Breastfeeding
In most women with stable PPCM, breastfeeding can be supported. However, in women with severe heart failure, the high metabolic demands of lactation and breastfeeding require consideration of prevention of lactation.
Counseling and subsequent pregnancies
Women are recommended to undergo six-monthly echocardiography until Left ventricular ejection fraction recovers to > 50% and annual visits for 10 years for women who remain stable after tapering of drug therapy for heart failure.
Echocardiographic findings of persistent LV dilatation or reduced myocardial strain may be present in some patients despite normal LV function. In such patients life-long heart failure therapy should be continued.
Heart failure symptoms are more frequent in women whose LVEF had not normalized before a subsequent pregnancy. Therefore, all patients with persistent LV dysfunction should be advised against future pregnancies.
All patients with previously diagnosed PPCM and their partners should receive careful counseling concerning the longer-term prognosis and undergo risk stratification if further pregnancies are considered.
Conclusion
PPCM remains an underdiagnosed peripartum disease due to its rarity. Women with symptoms consistent with heart failure should undergo clinical examination and cardiac biomarker testing, and echocardiography should be considered. Heart failure should be managed according to current guidelines, respecting the safety limitations during pregnancy and breastfeeding.
There is some evidence that bromocriptine might be helpful; a definitive trial is currently underway in the USA. Supportive therapy for heart failure should be initiated promptly and patient monitoring should be continued even after recovery of LV function.
Women with PPCM or who experienced PPCM in a previous pregnancy should be managed by an experienced multidisciplinary pregnancy heart team. This should include careful pre-pregnancy reassessment and counseling.